
Unique selling points
- High recovery rates: up to 90% of ammonia and 90% of phosphorous
- High product water quality which can be used in the chemical and fertiliser industry
- Low energy consumption
- Simple equipment and low maintenance cost
- Compact system with very small footprint
- No sludge production
- No start-up period required
Nitrogen and phosphorus removal and recovery via adsorption and ion exchange (IEX) - ammonium sulphate and calcium phosphate fertilizer production
Capacity
Pilot-scale demonstration - 10 m3/d nutrient stripped effluent
Description of the technology
Ammonia (NH4+) and phosphate (PO43-) are key components for fertiliser production and have a crucial role on the security of food production. On the other side, these nutrients are pollutants present in wastewater streams, and if untreated, these can have adverse effects on receiving water bodies such as algal blooms and toxicity problems. Recovery and reuse of these nutrients is highly beneficial for the environment and economy. Ion exchange (IEX) is one of the promising technologies for recovering nutrients from wastewater streams. One of the most promising ion exchangers for phosphorus removal from wastewater is the synthetic hybrid anion exchanger (HAIX) resin: a polymeric base where amorphous iron hydroxide nanoparticles (HFO-NP) have been dispersed. Two processes take place within the HAIX: the exchange of anions (Cl-, SO4-) between the polymeric base and the liquid phase and the adsorption of phosphorus (as divalent anion HPO42- and monovalent anion H2PO4 onto the HFO-NP via Lewis acid interactions. HAIX has been used to efficiently remove phosphorus from phosphorus-rich streams (including urine, municipal wastewater, surface waters, sludge dewatering liquors) but mostly in lab conditions and only recently, its feasibility was shown in SMART-plant as a tertiary treatment process.
Currently, the most used ion exchange media for ammonium removal are zeolites. These contain a crystalline aluminosilicate mineral within a framework of silica and aluminium tetrahedra linked by their corners through oxygen atoms. Cations present in the wastewater (usually NH4+, Na+, Ca2+, K+ and Mg2+) can penetrate inside cages and channels within the zeolite matrix and be exchanged with the surroundings. Engineered zeolites, like the one used in NEXT-GEN, have suffered chemical modifications to increase their selectivity towards ammonium.
Both ion exchange media must be regenerated when the desired effluent concentration is reached. The regenerant solutions used are 10% KCl and 2% NaOH to regenerate the synthetic zeolite and the HAIX, respectively. The nutrients can then be recovered from the saturated regenerants. Ammonia is recovered in the form of ammonium sulphate, (NH4)2SO4 via a membrane stripping system with sulphuric acid (H2SO4). In addition, for P recovery, PO43- is produced in the form of calcium phosphate, Ca5(PO4)3OH using a hydroxyapatite production system with the addition of hydrated lime to the NaOH regenerant. Ion exchange resins are regenerated using KCl and NaOH and thus reused in the IEX (Flow scheme of the technology).
Flow scheme of the technology
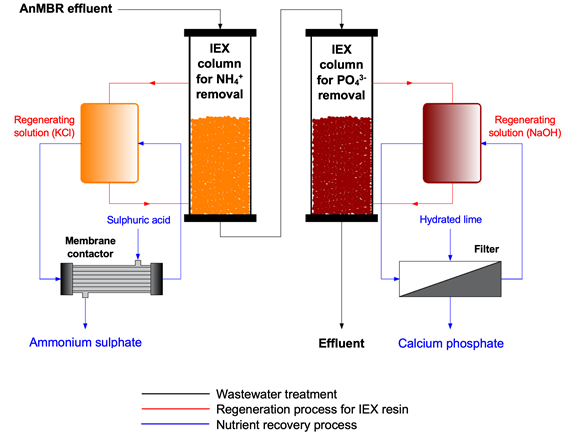
Pictures
Synergetic effects and motivation for the implementation of the technology
- Reduction of environmental impacts
Compared with the conventional aerobic process, anaerobic processes (i.e. anaerobic membrane bioreactor, AnMBR) could provide benefits of direct energy recovery, significant energy savings and additional treatment of excess sludge. However, AnMBR is ineffective at removing nutrients (ammonium and phosphate). High concentrations of nutrients in wastewater effluents can cause eutrophication with detrimental effects on the receiving waters. Coupling the existing treatment processes such as AnMBR with IEX processes can significantly reduce nutrient concentrations in the effluents and thus direct eutrophication emissions.
- Mining wastewaters as an alternative source of fertiliser production
Nutrient recovery from wastewater results in reduced production of energy-intensive fertilisers in many commercial agricultural sectors. As food production consumes a significant amount of fertiliser, an integrated AnMBR and IEX system can enhance the supply of adequate quality fertiliser to the market. This will boost resource recovery from wastewater streams, development of reuse/recovery technologies and production of acceptable fertiliser cost thus satisfying end-users.
- No energy requirement for nitrification or phosphorus removal
The ion exchange process allows for the selective removal and recovery of nutrients in their ionic state, without the need for any biological transformation. And thus 100% of the energy that would be used in the nitrification step and aeration of the stage of the biological phosphorus removal can be saved.
- No sludge production from nitrogen and phosphorus removal
In contrast to the microbial nitrogen removal via nitrification and denitrification, microbial phosphorus removal the ion exchange process does not rely on microorganisms and thus, does not produce additional sludge that must be disposed of later on.
Technology requirements and operating conditions
It is required to have nearly no solids and low organic matter. This is because solids will cause plugging in IEX columns and elevated organic matter concentration can cause biological fouling of the media.
Operating conditions
|
Technology |
Parameter |
Units |
Mean |
Reference |
|
|
Ion exchange |
IEX Influent |
Flow rate |
m3/day |
|
|
|
Temperature |
°C |
|
|||
|
IEX Effluent |
Flow rate |
m3/day |
|
||
|
Temperature |
°C |
|
|||
|
Membrane stripping system |
Regenerant NH4-N |
mg/L |
400-1000 |
||
|
Ammonia mass transfer, KLa |
L/h |
0.5-2 |
|||
|
N recovery rate |
% |
95 |
|||
|
H2SO4 consumption |
tons/year |
0.2 |
|||
|
Hydroxyapatite production system |
Regenerant PO4-P |
mg/L |
500-1000 |
||
|
Ca:P ratio |
- |
1.5-4 |
|||
|
P recovery rate |
% |
95 |
|||
|
CaOH consumption |
tons/year |
0.4 |
|||
Key performance indicators
Key performance indicators
|
Technology |
Parameter |
Units |
Min |
Max |
Reference |
|
|
Ion exchange (IEX) |
IEX Influent |
pH |
- |
|
|
|
|
|
BOD5 |
mg/L |
10 |
25 |
||
|
|
COD |
mg/L |
68 |
142 |
||
|
|
TSS |
mg/L |
0 |
0 |
||
|
|
NH4-N |
mg/L |
6 |
40 |
||
|
|
PO4-P |
mg/L |
2 |
7 |
||
|
|
SO4 |
mg/L |
0 |
0 |
||
|
IEX Effluent |
pH |
- |
|
|
||
|
|
BOD5 |
mg/L |
7 |
17 |
||
|
|
COD |
mg/L |
54 |
113 |
||
|
|
TSS |
mg/L |
0 |
0 |
||
|
|
NH4-N |
mg/L |
1 |
10 |
||
|
|
PO4-P |
mg/L |
0.5 |
2 |
||
|
|
|
SO4 |
mg/L |
0 |
0 |
|
|
|
N removal efficiency |
% |
75 |
83 |
||
|
|
P removal efficiency |
% |
71 |
75 |
||
|
Membrane stripping system |
N recovery rate |
% |
- |
95 |
||
|
(NH4)2SO4 production |
tons/year |
- |
0.53 |
|||
|
Hydroxyapatite production system |
P recovery rate |
% |
- |
95 |
||
|
Ca5(PO4)3OH production |
tons/year |
- |
0.63 |
|||
Links to related topics and similar reference projects
|
Nutrients recovery technology |
Reference |
|
Case study “Braunschweig”(NextGen) |
|
|
Case study “Altenrhein & Braunschweig” (NextGen) |
Publications
- Huang, X., Guida, S., Jefferson, B., & Soares, A., Economic evaluation of ion-exchange processes for nutrient removal and recovery from municipal wastewater, 2020

