
Unique selling points
- Easy operation
- Effectively remove ammonium
- Simple implementation
- Low energy consumption
- High adsorption capacity
- Regeneration process available
- Low cost
Description of the technology
The water treatment train proposed in this study case aims to treat water from an urban wastewater treatment plant to achieve the required quality for industrial reuse. The technology lineup includes a double-pass reverse osmosis system. To enhance energy efficiency, the plan is to replace the second osmosis step with zeolite adsorption, effectively reducing the ammonium content in the treated water. This innovative approach not only aligns with sustainability goals but also emphasizes the integration of zeolites (Fig. 1) for a more environmentally friendly and resource-efficient industrial water treatment process.
Exploring and adopting sustainable technologies is essential not only for enhancing the efficiency of water purification processes but also for minimizing the ecological impact and contributing to a more sustainable future. In this context, the quest for alternative solutions becomes a key focus to address the energy challenges posed by commonly used techniques and pave the way for more environmentally friendly water treatment practices.
Zeolite emerges as a promising alternative, offering efficient adsorption of heavy metals and compounds through ion exchange. This innovative approach not only enhances water quality but also promotes sustainable and efficient resource management practices, presenting a viable solution to the pressing issues faced by water treatment plants.
Zeolites have been widely used as catalysts, ion exchangers, and adsorbents since their industrial breakthrough in the 1950s and continue to be state-of the-art adsorbents in many separation processes (Perez-Botella et al. 2022). Furthermore, their properties make them materials of choice for developing and emerging separation applications. The use of zeolites as adsorbents stems ultimately from their microporosity and regular pore size (Perez-Botella et al. 2022). They are mainly composed of aluminosilicates with a three-dimensional structural composed by Al-O and Si-O tetrahedra networks (Wen et al. 2013, Xue et al. 2018).
Zeolite has an excellent ion exchange capacity, and its cation exchange ranking is Cs+ > Rb+ > K+ > NH4+ > Sr+ > Na+ > Ca2+ > Fe3+ (Lin et al. 2013, Pan et al. 2019).
Flow scheme of the technology
The following diagram (Fig. 1) shows the process where the adsorption system with zeolites is incorporated to replace the second pass of reverse osmosis.
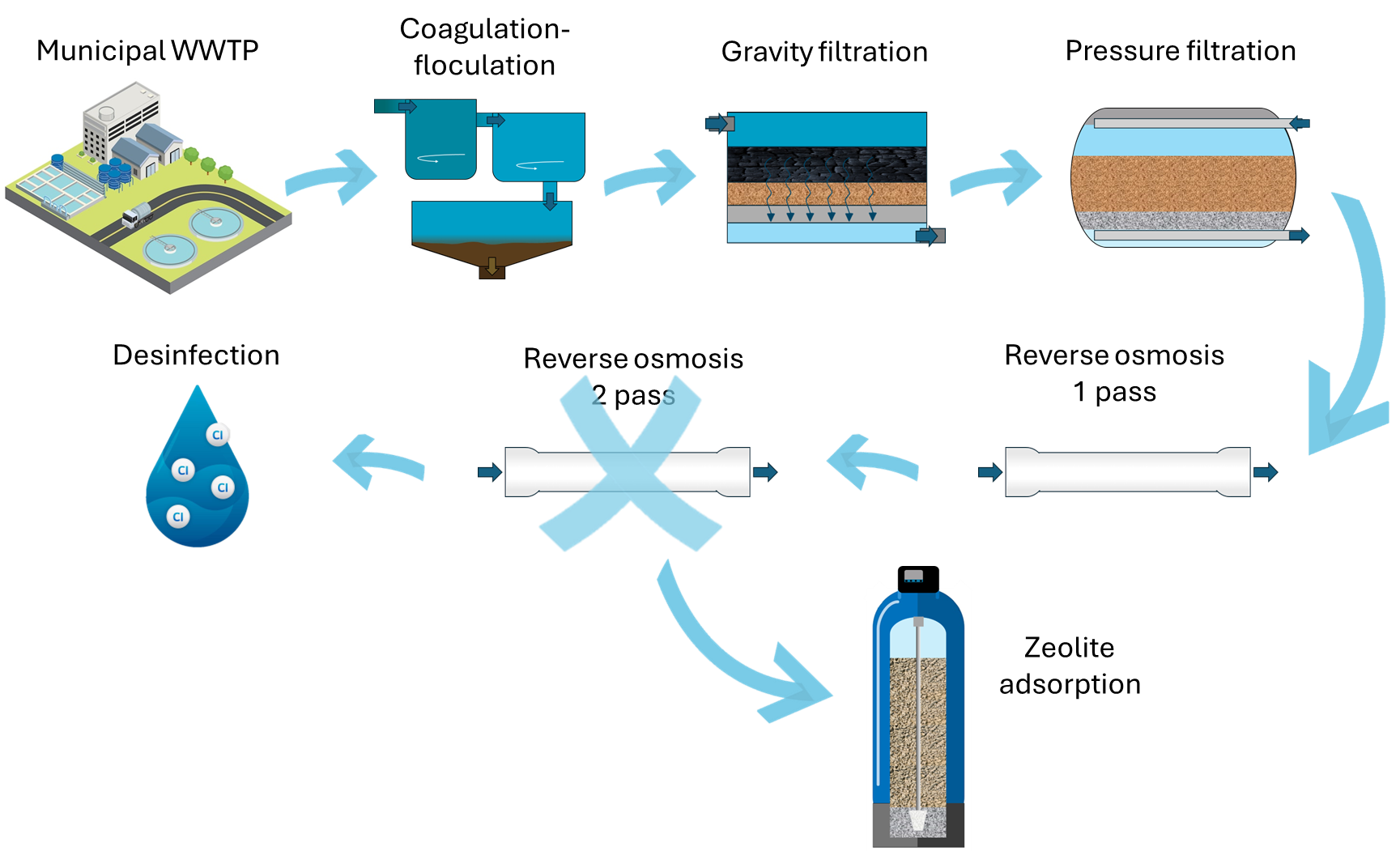
Fig. 1. Water treatment process including the zeolite adsoption system
The zeolite adsorption column is associated with a regeneration tank containing NaCl 10%, as can be seen in Fig. 3 and in the system images (Fig. 2).
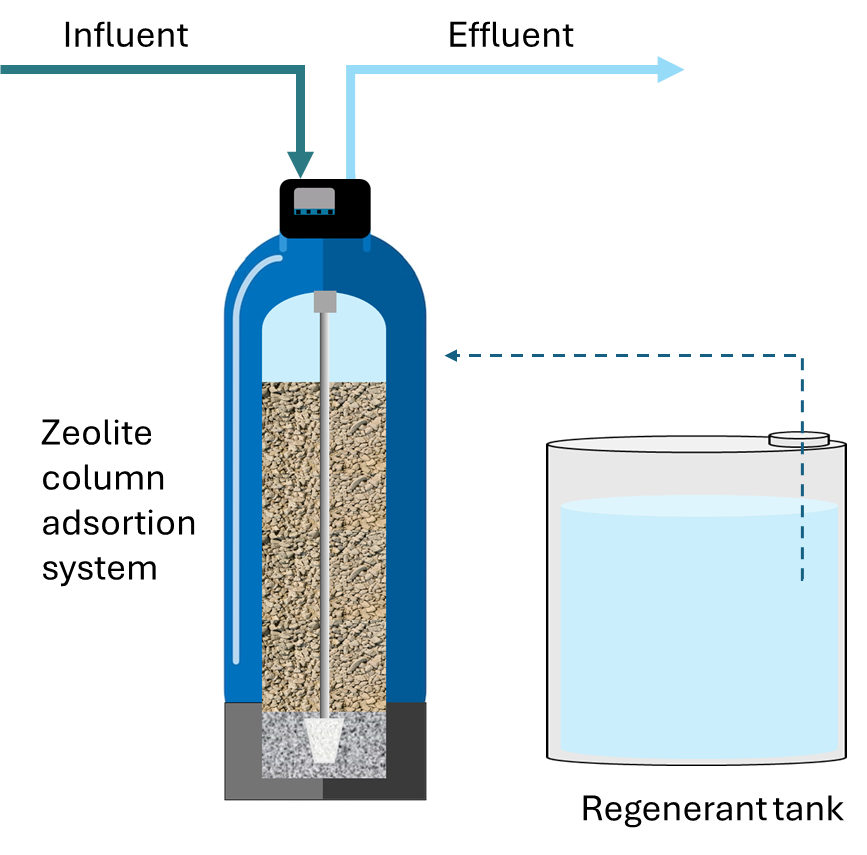
Fig. 2. Scheme of the zeolite column adsorption system
The following picture (Fig. 3.) shows the zeolite adsorption system installed in CS1 pilot plant from ULTIMATE project.

Fig. 3. Zeolite column adsorption system
Synergetic effects and motivation for the implementation of the technology
The reuse of industrial water comes with its set of challenges, particularly concerning the presence of ammonium ions (NH4+), which have been identified as a significant factor influencing corrosion. According to a study conducted by Arjmandi et al., 2019, the weight of influential parameters reveals that ammonium, along with dissolved oxygen and iron, exerts the maximum effect on corrosion.
The need for sustainable water resource management has driven the reuse of industrial water, especially in cooling systems. However, Eslamian et al. (2013) caution that despite the suitability of wastewater reuse for sustainable water management, there are associated risks. Among these risks, the impact of ammonium on corrosion stands out.
Efficient techniques for reducing NH4+ concentrations in reused industrial water, as zeolite adsorption, are crucial to mitigate corrosion risks. Importantly, these techniques should also align with energy efficiency standards, advocating for methods with lower energy consumption compared to conventional processes like reverse osmosis (RO).
Balancing the imperative for water resource sustainability with the necessity to curb corrosion highlights the need for innovative, low-energy methods to effectively lower NH4+ concentrations in industrial water reuse. Addressing these challenges will not only enhance the longevity of infrastructure but also contribute to the overall sustainability goals of industrial water management.
Technology requirements and operating conditions
The effective implementation of ammonium adsorption on zeolites requires meeting specific technological requirements and maintaining optimal operating conditions. These requirements ensure high efficiency, sustainability, and cost-effectiveness in the adsorption process.
Technology Requirements
- Zeolite selection and preparation
- High CEC zeolites: Choose zeolites with high cation exchange capacity (CEC) such as clinoptilolite, mordenite, or chabazite.
- Pre-treatment: Depending on the source and intended application, zeolites may need pre-treatment processes like washing, acid treatment, or ion exchange to enhance their adsorption properties.
- Particle size optimization: Crush and sieve zeolites to obtain the appropriate particle size that maximizes surface area and minimizes diffusion resistance.
- System Design
- Batch systems: Suitable for small-scale applications or initial testing phases. Requires mixing tanks with controlled agitation and temperature.
- Continuous flow systems: Includes fixed-bed or fluidized-bed reactors designed to handle larger volumes. These systems require precise control overflow rates and contact time.
- Column design: For fixed-bed systems, columns should be designed to prevent channeling and ensure uniform flow distribution. Packed bed columns are commonly used for this purpose.
- Regeneration system
- Regeneration units: Equip the system with units capable of regenerating spent zeolites using saline solutions or other regenerants.
- Recovery and reuse: Implement processes to recover and reuse regenerants if possible, to reduce operational costs and environmental impact.
- Monitoring and control systems
- Sensors and instrumentation: Install sensors for real-time monitoring of ammonium concentrations, pH, temperature, and flow rates.
- Automation and control: Automated control systems to maintain optimal operating conditions and adjust parameters as needed.
- Waste management systems
- Spent zeolite handling: Design systems for the safe handling, storage, and disposal of spent zeolites.
- Effluent treatment: Include units for treating effluents from the regeneration process to comply with environmental regulations.
Operating Conditions
- Solution pH
- Optimal range: Maintain the solution pH within 6.5 to 8.5. Ammonium adsorption is most effective within this range, as extreme pH levels can reduce ion exchange efficiency and damage the zeolite structure.
- Temperature
- Control temperature: Typically, ambient temperature is suitable, but maintaining a consistent temperature can improve adsorption kinetics. For specific applications, slightly elevated temperatures may enhance the process but should not exceed the thermal stability of the zeolite.
- Contact time
- Adequate residence time: Ensure sufficient contact time between the zeolite and ammonium-containing solution. Batch processes should allow enough time for equilibrium to be reached, while continuous systems should be designed to provide adequate residence time.
- Flow rates
- Controlled flow rates: In continuous systems, regulate flow rates to optimize contact time and prevent channeling. This ensures maximum utilization of the zeolite’s adsorption capacity.
- Agitation and mixing
- Uniform Mixing: In batch systems, provide adequate agitation to ensure uniform distribution of ammonium ions and maximize contact with zeolite particles. In continuous systems, design the flow to prevent dead zones.
- Competing ions
- Minimize interference: Control the concentration of competing cations (e.g., Na+, K+, Ca2+, Mg2+) in the solution to minimize their impact on ammonium adsorption efficiency.
- Regeneration frequency
- Regular Regeneration: Determine and implement a regular regeneration schedule based on the zeolite’s adsorption capacity and the ammonium load. Regeneration frequency depends on the initial ammonium concentration and the zeolite’s capacity.
- Safety and environmental compliance
- Safety Protocols: Ensure that all handling, processing, and regeneration activities adhere to safety standards to protect workers and the environment.
- Environmental regulations: Comply with local and national regulations regarding wastewater treatment, effluent discharge, and disposal of spent materials.
Table 1. Adsorption capacities and other parameters for ammonium removal by natural zeolites and clays (Huang et al., 2018).
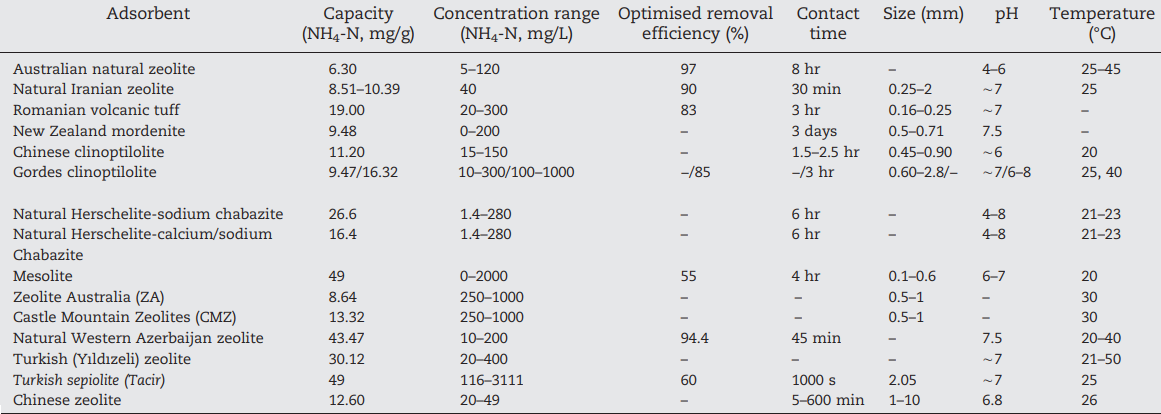
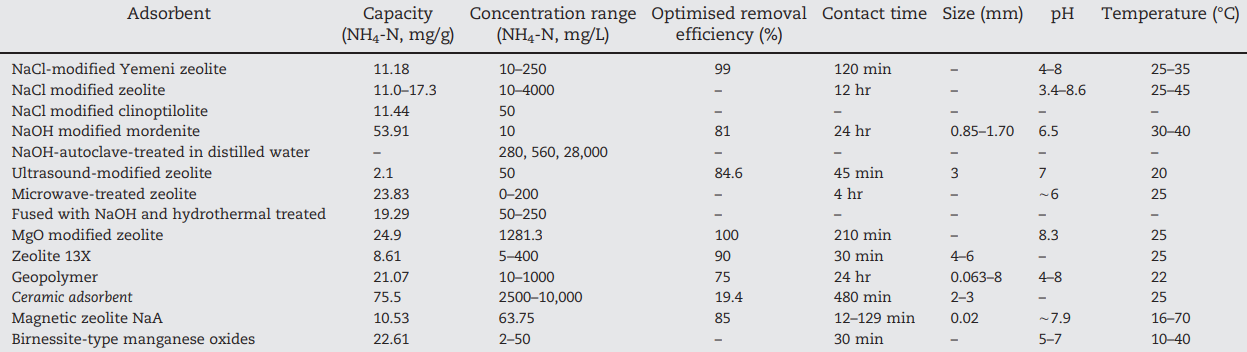
Table 2. Adsorption capacities and other parameters for ammonium removal by synthetic zeolites (Huang et al., 2018).
Key performance indicators
Ammonium adsorption on zeolites is a critical process in water treatment and environmental management. The key parameters and indicators that influence and measure the effectiveness of ammonium adsorption on zeolites include:
1. Zeolite type and structure
- Framework type: Different zeolite frameworks (e.g., clinoptilolite, mordenite, and chabazite) have varying affinities for ammonium ions due to differences in pore size and structure.
- Si/Al ratio: The silicon-to-aluminum ratio affects the cation exchange capacity (CEC) of the zeolite. A higher aluminum content generally increases the number of exchange sites.
- Pore size and volume: The physical structure, including pore size and volume, determines the accessibility of ammonium ions to the exchange sites.
2. Adsorption capacity
- Adsorption capacity: A critical parameter indicating the amount of ammonium ions that a given amount of zeolite can adsorb. It is typically measured in meq/g (milliequivalents per gram).
3. pH of the solution
- Optimal pH range: The efficiency of ammonium adsorption on zeolites is influenced by the pH of the solution. Generally, a neutral to slightly alkaline pH (around 6.5 to 8.5) is favorable for ammonium ion uptake.
4. Concentration of ammonium ions
- Initial concentration: Higher initial concentrations of ammonium in the solution can increase the adsorption capacity until the zeolite reaches saturation.
- Equilibrium Concentration: The concentration of ammonium ions remaining in solution after equilibrium provides insights into the adsorption efficiency.
5. Temperature
- Adsorption Kinetics: Temperature can affect the rate of adsorption and the overall capacity. Higher temperatures often increase the adsorption rate due to enhanced ion mobility but may also affect equilibrium adsorption capacity.
6. Contact Time
- Equilibrium time: The time required for the ammonium ions to reach equilibrium adsorption on the zeolite. This influences the design and operation of treatment systems.
7. Competing Ions
- Presence of other cations: Ions such as Na+, K+, Ca2+, and Mg2+ can compete with ammonium for exchange sites, affecting the adsorption capacity.
8. Particle size
- Surface area and diffusion path: Smaller particle sizes provide a larger surface area for adsorption and shorter diffusion paths, potentially enhancing adsorption rates.
9. Regeneration ability
- Regeneration and reusability: The ability to regenerate zeolites (e.g., by washing with saline solution) and their performance after multiple adsorption-desorption cycles are important for practical applications.
10. Adsorption Isotherms
- Langmuir and Freundlich isotherms: These models describe the adsorption equilibrium and help predict the adsorption capacity of zeolites under different conditions.
11. Adsorption Kinetics
- Pseudo-first order and pseudo-second-order models: These kinetic models describe the rate of ammonium adsorption and help in understanding the adsorption mechanism.
12. Thermodynamic Parameters
- Gibbs free energy (ΔG): Indicates the spontaneity of the adsorption process.
- Enthalpy (ΔH) and entropy (ΔS): Provide insights into the nature (endothermic or exothermic) and randomness of the adsorption process.
By considering these parameters and indicators, the effectiveness of ammonium adsorption on zeolites can be optimized for specific applications in water treatment and environmental remediation.


